Haematopoietic stem cells are a type of adult stem cell found in the blood and bone marrow. Haematopoietic stem cells are able to generate new blood and immune cells. Autologous haematopoietic stem cell transplant (AHSCT, also known as bone marrow transplant) used for multiple sclerosis (MS) is similar to the chemotherapy treatment used to treat blood cancers – chemotherapy is used to eliminate the immune system, and then the blood and immune system are restored, in this case, using the patient’s own (autologous) blood stem cells. The aim is to ‘re-boot’ the immune system so that the self-reactive immune cells that are attacking an individual’s nervous system are removed, and replaced with the regenerated immune system, which is thought to be more ‘self-tolerant’ and less likely to continue attacking the body. This type of stem cell treatment is not thought to contribute to repair of the nervous system, but is primarily used to restore the blood and immune system following the immuno-suppressive chemotherapy treatment.
AHSCT is an intensive form of treatment which involves a number of steps, including:
Supportive medical treatment is provided in the immediate period following transplant when there is a high risk of infection and bleeding disorders due to the intense immune suppression.
MS Australia is supporting a registry to closely follow the progress of people with MS undergoing the AHSCT procedure. If you have been, or are going to be, treated with AHSCT for MS, and would like to have your data included in the registry, please contact us or visit the FAQ page.
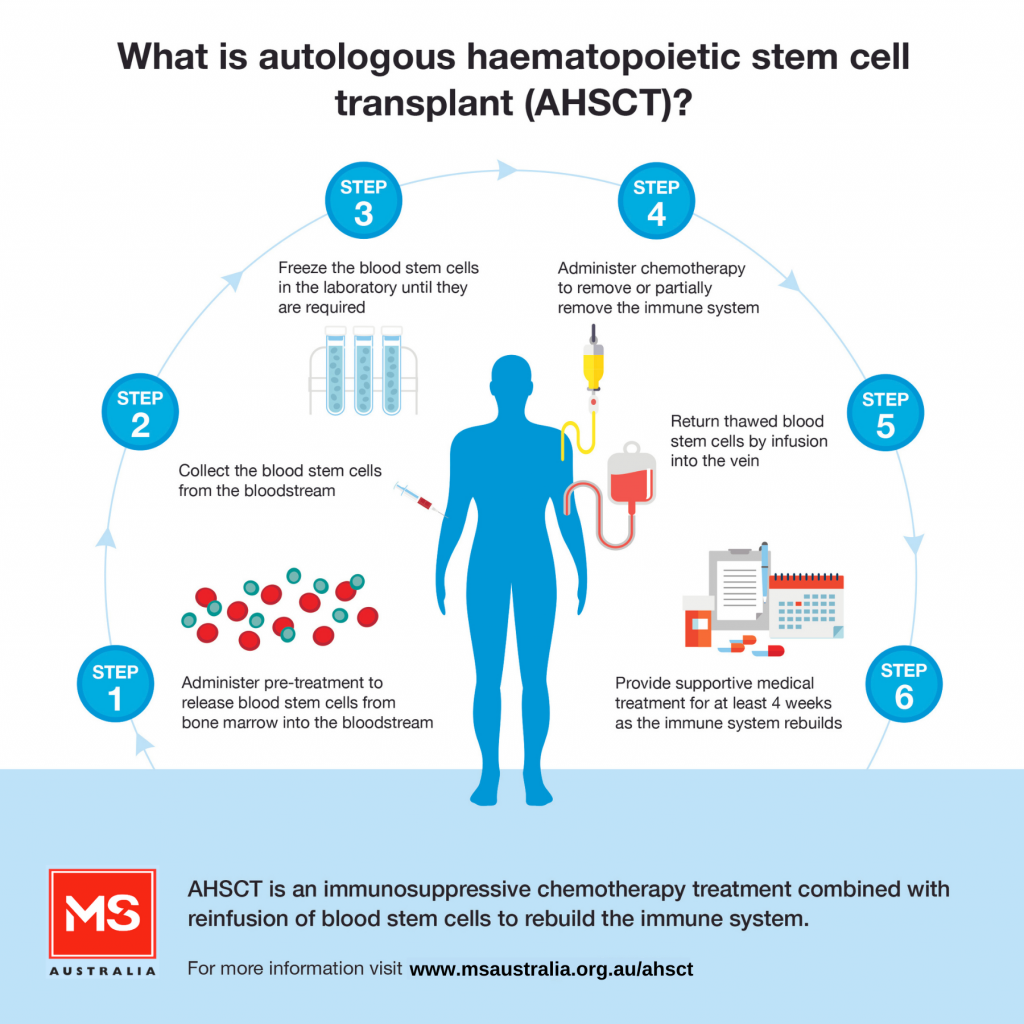
Autologous haematopoietic stem cell transplant (AHSCT) is an immunosuppressive chemotherapy treatment combined with reinfusion of blood stem cells to help re-build the immune system. It is increasingly being used to treat a small group of carefully selected people with multiple sclerosis (MS) in Australia and internationally.
After many years of exploration and mixed results, there have recently been positive steps forward for people with MS considering AHSCT. As an invasive treatment with significant concerns and potential side effects, AHSCT is not for the faint-hearted, and not something to consider for most people with MS. However, the evidence is mounting that there is a very distinct group of people with MS for whom AHSCT is more likely to work well. AHSCT is essentially a very powerful anti-inflammatory treatment, so it stands to reason that it might be most effective in situations where there is a lot of active inflammatory activity, which we see most in relapsing remitting MS (RRMS) with relapses and active inflammatory lesions on an MRI, and less in progressive MS with no inflammatory features. If no inflammation is present, then AHSCT may not be very helpful, as the stem cells involved in this treatment cannot repair the previous damage to nerves and myelin.
Let’s consider some of the reasons why AHSCT needs to be well thought out and directed to those in whom it might be the most effective and safe.
Most disease modifying therapies (DMTs) for MS are relatively simple to administer in the big scheme of things. Obviously, taking a capsule or tablet is the least troublesome. Training for and administering self-injections at home push the difficulty factors up another notch in terms of effort and inconvenience. Needing to go into a hospital day only clinic or ward and having an intravenous (IV) line inserted for an infusion takes it up yet another level of inconvenience and complexity. There is not too much distress involved in any of these treatments most of the time; they are relatively free of too much discomfort. In addition to these three methods of taking DMTs, there are also safety monitoring responsibilities such as regular blood tests, eye tests for others and extra MRIs, depending on which therapy has been prescribed. But for many people living with MS, the inconvenience of these treatments is deemed worthwhile by them because the treatments work to reduce their relapses and disease progression and allow many facets of life to return to normal, or close to it. The benefits outweigh both the risks and the amount of bother it brings (the “bother factor”). For each step up the ladder, the MS healthcare team has had to carefully consider benefit and risk and what they feel is justified according to each individual circumstance. Keeping you as disease-free as possible, and as safe as possible, is paramount.
Now, we get to AHSCT. This is a whole other level of potential risk, suffering and bother than we have seen in MS previously. It really has created a whole new platform to consider for both people with MS, their loved ones and their MS healthcare teams and has not been taken lightly by the neurology, immunology and haematology specialties who have worked together to bring AHSCT to the treatment arena. AHSCT requires much preparation and there is more than reasonable potential for suffering and distress during and after the procedures. However, for people living with very active inflammatory MS which is not responding to standard DMTs, AHSCT might be the saviour to future quality of life, and the chance to live the type of life they had planned out.
The range of effects that AHSCT causes we have not previously seen in MS treatments before, and this has required careful consideration and planning. This includes the effects of the large-dose chemotherapy in general and the massive inherent infection risk, as well as the fertility effects on both males and females, as the testes and ovaries are both affected by the treatment. After stem cell harvest and the very large dose of chemotherapy to destroy the faulty immune cells, the risk of infection is very high, and patients must isolate for several weeks to protect themselves. The good news is that since 2005, the risk of death from AHSCT has gradually decreased, with recent research reporting a risk of less than 1%, compared to about 3% a decade ago. This is due to several reasons, including improved chemotherapy regimens, improved prevention and treatment of infections, the knowledge gained from past patients and improved selection of the patients most likely to recover well and to benefit from the procedure. Additionally, many MS specialists and scientists from all over the world contribute their patient progress information to registries to gather more knowledge and to help build a profile of the type of MS patients who benefit the most from AHSCT. This helps to keep the risk to benefit balance in check and to continually educate the MS community on the best and safest practice. For those living with active inflammatory MS not responding to standard treatments, AHSCT could be part of the treatment discussion they need to consider to change their current MS pathway. Read on to find out more.
MS Australia has worked with Australian haematologists, neurologists and members of the research and MS communities to develop a position statement on the use of AHSCT for MS with guidance on its possible place within the range of treatment options for MS in Australia. This position statement has been developed with reference to the current data published in the international peer-reviewed scientific literature – an overview of this data is discussed below.
You can read the MS Australia AHSCT Position Statement here.
MS Australia (with initial support from the MS Society of Western Australia), is coordinating and funding the Australian AHSCT MS Registry – this registry is overseen by a steering committee of Australian haematologists and neurologists, and aims to gather data and monitor the outcomes of Australians treated with AHSCT for MS. This important Registry will add to the data being gathered world-wide on treatment effects, patient characteristics and outcomes, and long-term prognosis. Together with the international evidence, the Registry aims to assist in better understanding how AHSCT can be used as part of the range of treatments available for MS as safely as possible and in the right circumstances. Currently, the Registry is collecting and analysing this data, including longer-term outcomes.
If you have been, or are going to be, treated with AHSCT for MS, and would like to have your data included in the Registry, please contact us at enquiries@msaustralia.org.au. We will put you in touch with the Australian AHSCT MS Register Research Fellow who will provide you with more information and will need your consent to contact your doctor(s) to access clinical notes and data.
Please note that this Registry is only for the follow up of people who have received, or are receiving, AHSCT under the care of their treating doctors. MS Australia is unable to assist people to access this form of treatment. People with MS are urged to contact their specialist MS physician for more information.
The intense immune suppression associated with chemotherapy can leave people vulnerable to severe infections and bleeding in the immediate period following the procedure, which may be life-threatening and require intensive medical support. However, advances in supportive care have seen the mortality risk decline in recent years. Experience with AHSCT in Australian patients, the majority of whom are treated for blood cancers, suggests that AHSCT has a transplant related mortality (TRM) rate of around 1% in the immediate 100 days following transplant4. The large European case series of AHSCT in people with autoimmune diseases recorded an average TRM of 1.3% for people with MS treated between the years 2001 and 20077. Most recently, a study published in 2021 which looked at over 500 patients treated between 2003 and 2019 in a single American hospital recorded a TRM of 0.19%24. A number of different chemotherapy regimens were included in these studies7, 24. Some recent, but smaller, published studies have not recorded any deaths6,8,9,10,11, 23. No transplant related deaths have been recorded at the Australian centres that have provided this treatment to people with MS 4, 25.
Despite the decline in mortality from the procedure, the risk of infections from bacteria, viruses and fungi, that would not normally affect people with an intact immune system, remains significant. These infections can result in prolonged hospital stays and, in some cases, may be associated with worse neurological outcomes5,10, 20. Experience with treating blood cancers over many years, as well as autoimmune disorders, suggests that there may also be longer-term adverse effects of HSCT, such as effects on other organs, including heart liver, kidney and bone health, reduction in fertility, secondary cancers and secondary autoimmunity5. In a USA study, the use of anti-thymocyte globulin (ATG) in the conditioning regimen was associated with a 2% risk of secondary autoimmunity, particularly of the thyroid15.
The risks of AHSCT will differ depending on the form of chemotherapy used and this should be discussed with the treating haematology centre.
In most of the studies reported above there has been no evidence presented for AHSCT treatment causing an exacerbation or escalation of MS progression, although the risk of MS relapses is known to be higher in association with infections20 which may occur during the period of immune suppression following AHSCT. In the Chicago case series discussed above, the authors noted that sustained fever during hospitalisation for HSCT was associated with higher disability several years later in comparison to those who did not experience sustained fever10. In most of the studies, there have been a small proportion of patients who continue to experience MS disease activity following treatment, but studies have not documented changes in the frequency of relapses or MRI lesions from the pre-transplant to post-transplant period in those who do not respond to AHSCT following treatment. Canadian and Italian studies have both shown that there is an initial rapid loss in brain volume following AHSCT, but that after this period brain shrinkage then continued at a similar or slower rate to that seen prior to AHSCT12, 13.
Some researchers have examined the profile of immune cells found in the blood of people with MS following AHSCT. These studies have suggested that it is not long-term immune suppression that accounts for the effects of AHSCT on MS, but rather a resetting of the profile of different types of immune cells in the blood. Studies have shown that white blood cell numbers return to pre-transplant levels within two years following high dose chemotherapy14,18. These studies, as well as others, show that new T cells (a type of immune cell that protects the body from infection) are generated and these cells demonstrate a more diverse range of subtypes. While T cells that react to components of myelin may not completely disappear following AHSCT, the balance appears to be shifted to a more ‘self-tolerant’ and less inflammatory status14, 16, 17, 18. However, further research is needed to fully understand the long-term effects that AHSCT has on the immune system.
Currently, the treatment is provided in Australia through three observational clinical trials, at St Vincent’s, Sydney (more information here), Austin Health, Melbourne (more information here) and The Alfred, Melbourne (more information here) with similar entry criteria. These trials have strict eligibility requirements approved by a hospital ethics committee and may only apply to limited numbers of patients with MS. It is for this reason that all sites require patients to be referred to them by a neurologist including a detailed clinical history and MRI findings. These centres and other Australian centres that are providing the treatment on a case-by-case basis are also contributing data to the Australian MS AHSCT Registry.
In a study comparing the effectiveness of AHSCT with natalizumab for people with progressive MS, researchers found no significant difference in disability progression or improvement between the two treatments. Conducted across seven AHSCT MS centres and using data from the registry, MSBase, the study matched 39 people treated with AHSCT with 65 people treated with natalizumab based on various criteria, including sex, age, and MS history. Over a period of up to four years, both groups showed similar hazards for disability worsening and improvement, as well as relapse activity. The AHSCT group experienced several complications, including febrile neutropenia, serum sickness, and the need for intensive care in some cases, but no treatment-related deaths were reported. The results suggest that AHSCT may not be more effective than natalizumab in controlling disability in people with progressive MS with advanced disability and low relapse activity.
This study evaluated the outcomes of AHSCT for the treatment of MS in a real-world setting24. The researchers analysed data from 414 individuals with relapsing remitting MS (RRMS) and 93 with newly diagnosed secondary progressive MS (SPMS) who underwent AHSCT at Northwestern University, Chicago between July 2003 and October 2019.
The results showed that the overall survival rate after AHSCT was 98.8%, with one treatment-related death and one case of bacterial infection. However, secondary autoimmune diseases were observed in some individuals after AHSCT, including idiopathic thrombocytopenia (ITP) and hypo/hyperthyroidism. The incidence of ITP was higher in individuals treated with alemtuzumab compared to other regimens.
The study found that the 5-year relapse-free survival rate was 80.1% for RRMS and 98.1% for SPMS, while the 4-year progression-free survival rate was 95% for RRMS and 66% for SPMS. In RRMS, the disability status as measured by the Expanded Disability Status Scale (EDSS) significantly improved at each follow-up visit after AHSCT. However, in SPMS, the EDSS score only significantly improved at 1 year but not thereafter. Based on these findings, the researchers observed that AHSCT is effective as a one-time therapy for individuals with RRMS. However, the benefits of AHSCT appear to be relatively less pronounced for individuals with newly diagnosed SPMS. This study offers real-world evidence supporting the application of AHSCT as a potential treatment option for people with MS, particularly those with RRMS.
In a retrospective study at a single academic centre in Florence23, researchers investigated the effectiveness of AHSCT treatment compared to treatment using low dose immunosuppression with cyclophosphamide (Cy) in secondary-progressive MS (SPMS)
This study included 93 individuals with SPMS, with 31 receiving AHSCT and 62 receiving Cy treatment. The researchers analysed the data to assess the impact of the treatments on relapse activity, disability progression, and disease activity.
The results showed that AHSCT was more effective in reducing relapse activity compared to Cy treatment. At the 5-year mark, the AHSCT group had a 100% survival rate without relapses, while the Cy group had a 52% rate. However, there were no significant differences between the two groups in terms of disability progression or disease activity.
The study suggests that AHSCT may be a more effective treatment option for reducing relapse activity in people with SPMS. However, both treatments showed similar effects on disability progression, indicating that non-inflammatory neurodegeneration may play a more significant role in SPMS-related disabilities compared to inflammation. This study has some limitations, and further research is needed to validate these findings to better understand the long-term effects of AHSCT in SPMS.
Published in February 202121, this study looks at 20 people who had been classified as having ‘aggressive’ MS and whether AHSCT was successful as a first line therapy (i.e. being the first treatment given to these people). MS is generally classified as aggressive when one of the following is experienced; rapid accrual of disability, two or more relapses with incomplete recovery in a year, three or more MRIs showing new lesions. In this study there were three different groups of drugs used as the chemotherapy treatment in the procedure (called a “conditioning regimen”) shortened to Bu-Cy-ATG, BEAM-ATG and Cy-ATG. The average follow-up period was 30 months. None of these patients experienced confirmed disability progression. Disability scores of 13 patients plateaued after the initial 6 months, whereas scores of five patients continued to improve beyond this period. 17 patients had no new MRI lesions following AHSCT. Three patients had new lesions on their first MRI scans. There were the expected adverse events with any type of AHSCT using chemotherapy agents, including low white blood cell count, fever, low platelet counts, anemia, and diarrhea. In total, 20% of patients developed thyroid issues following AHSCT. There were no reported cancers from the study. This is a small study of people with aggressive MS, the study design does provide a solid base for future larger studies.
In this study22, researchers followed 210 people who had AHSCT from 20 different Italian treatment centres for an average of 6.2 years, to try and understand the long-term effectiveness of AHSCT for MS. The study included people who had undergone AHSCT between 1997 and 2019. Of the 210 people followed, 122 had relapsing remitting MS, 86 had secondary progressive MS, and two had primary progressive MS. Three people died within 100 days of their treatment and this was considered to be related to the AHSCT. Overall, at five years after AHSCT, 79.5% of participants had no disability worsening, at ten years this fell to 65.5%. When the participants were separated into groups based on their type of MS, those with relapsing-remitting MS showed a greater response to AHSCT. Those with relapsing remitting MS and no disability worsening was 85.5% at five years and 71.3% at ten years. However, for study participants with progressive MS, 71.0% had no disability worsening after five years and 57.2% after ten years. The researchers also looked at the relapse rate and showed that 78.1% of people with relapsing remitting MS remained relapse free after five years following AHSCT. After ten years, this dropped to 63.5%. Besides relapses, another measure of the effectiveness of a treatment is NEDA. NEDA stands for “no evidence of disease activity” and is essentially a measure which combines three factors: relapses, signs of disease activity by MRI and signs of disability worsening on examination. When the researchers used NEDA to measure how effective AHSCT was in people with relapsing remitting MS, the likelihood of being NEDA at five years was 62.2%, and 40.5% at ten years. In the group with progressive MS, NEDA status estimates were lower at 50.8% and 37.3% at five and ten years, respectively. This study is important to our understanding of the place of AHSCT in MS care because of the comparatively large study numbers, the depth of information collected, and the long period of time the participants were followed for.
An Australian AHSCT trial, published in May 2019, looked at the safety and effectiveness of AHSCT (using the chemotherapy regimen of BEAM-ATG) in 20 people with relapsing remitting MS and 15 people with secondary progressive MS over a three year period. The trial showed that 60% of participants had no evidence of disease activity in the three years following treatment (70% in relapsing remitting MS). 73% of participants had no disability progression during the follow-up period. 37% of participants showed significant improvements in their disability levels, of those showing improvement, all bar one had relapsing remitting MS. However, the disease continued to progress in eight participants during the follow-up period; two with relapsing remitting MS and six with secondary progressive MS. Participants experienced the expected side effects, including ulcers in the digestive tract, nausea and hair loss. 34% of participants required red blood cell transfusions and 49% required platelet transfusions following treatment.
The Multiple Sclerosis International Stem Cell Transplant (MIST) AHSCT trial, published in January 2019, compared the effect of non-myeloablative AHSCT and standard disease modifying therapy on MS progression in 110 participants with relapsing remitting MS over a one to five year period. The trial showed that the people treated with AHSCT showed improvement in disability, whereas those receiving standard therapies showed worsening disability. After one year of follow-up, 1.92% of AHSCT-treated participants had experienced relapses compared to 64.3% of participants receiving standard therapies. After five years of follow-up, 15.4% of the people treated with AHSCT had experienced relapses compared to 85.2% in the standard therapy group. There was also a reduction in the total volume of existing MRI brain lesions in the AHSCT group compared to an increase in the standard therapy group. Measurements of quality of life also showed improvements in the AHSCT group compared to the standard therapy group. There were no deaths and no participants who received AHSCT experienced disabling or potential life-threatening events.
A Canadian AHSCT trial published in June 2016, looked at the safety and effectiveness of AHSCT using a myeloablative busulfan, cyclophosphamide and ATG regimen in 24 people with MS (12 with relapsing remitting MS and 12 with secondary progressive MS). The participants were followed up over an average period of 6.7 years following treatment (range of 3.9-12.7 years). The trial showed that 69.6% of the participants remained event free for the three years following treatment (defined as no relapse, no change in disability level and no new MRI lesions). There were also no signs of new MRI lesions or relapses in all participants during the extended follow-up period. 70% of participants experienced no further disability progression after the extended follow-up period. Brain shrinkage, which is normally accelerated in people with MS, decreased to a rate similar to the rate seen in normal ageing.
One participant died due to transplantation-related complications. As expected, all participants experienced some toxic side effects and a reduced white blood count for a short time.
The ASTIMS trial6, published in March 2015, compared AHSCT (using BEAM-ATG chemotherapy) to mitoxantrone (a potent immunosuppressive treatment sometimes used for the treatment of MS) in 21 people with relapsing remitting or secondary progressive MS. The trial showed AHSCT to have a significantly greater effect on reducing relapses and evidence of lesions on MRI compared to mitoxantrone. This trial did not show any reduction in disability. Eight of the 11 people treated with mitoxantrone experienced adverse events. All of the nine people treated with AHSCT, experienced expected adverse events, including low white blood cell count, fever, low platelet counts, anemia and diarrhea. No transplant related deaths were recorded, however, four of the nine AHSCT patients experienced severe adverse events (two of which were described as life-threatening) including sepsis, severe infections and late or failed engraftment of the re-infused stem cells.
Dr Richard Burt and colleagues at Northwestern University, Chicago published their case series of 123 people with relapsing remitting MS and 28 people with secondary progressive in January 201510. Participants were treated with the non-myeloablative Cyclophosphamide-ATG regimen (129 patients) or with Cyclophosphamide-Alemtuzumab (22 patients). The study found that after one year, 57 of 112 patients showed some evidence of improvement in disability scores while 19 showed evidence of disease progression. 80% of the 36 participants who were followed for 4 years were relapse-free and 87% showed no progression of disability. At the time of publishing, only 27 patients had been followed for 5 years, but 14 of them showed evidence of improvement and 4 showed evidence of disease progression, the rest remained stable.
When relapsing remitting and progressive MS cases were analysed separately, the study identified that the improvements in disability were strongest in the participants with relapsing remitting MS with duration of disease less than ten years. There were no transplant related deaths. Four patients developed late reactivation of herpes zoster infection (shingles). Severely low platelet counts and thyroid dysfunction was recorded in 6.9% of patients treated with the Cyclophosphamide -ATG protocol and 22.7% of patients treated with the Cyclophosphamide -Alemtuzumab protocol.
This research group is now leading an international multi-centre randomised clinical trial of AHSCT in comparison to other MS therapies. The trial, known as MIST, has sites in USA, UK, Sweden and Brazil (more information here). Encouraging results for three of the patients from the UK arm of the MIST trial have been featured in the media, however, we await with great interest the full results of the trial, which has enrolled at least 100 patients and is due to be completed in 2017.
In February 2015, clinicians at the Pirogov National Medical Surgical Centre in Moscow published a case series of 99 patients with MS (43 relapsing remitting and 56 progressive MS) treated with a reduced-intensity BEAM-like regimen at the centre11. 83.3% of relapsing remitting patients remained ‘event free’ after an average of 4 years follow-up and 75.5% of progressive patients remained ‘event free’. Of patients followed up for 8 years, 16.7% showed progression of disease. Of the 64 patients followed for three years or more, 47% were described as having improved disability scores by at least 0.5 points. No deaths were recorded, but the paper did not include details of adverse events or the number of patients affected. We are aware that many overseas patients including Australians are also treated at this centre and may receive a conditioning regimen that differs from the one described in this publication. Details of conditioning regimens used for overseas patients at this centre and treatment outcomes have not yet been published in the medical literature.
A Swedish study published in February 20148 enrolled 48 people with MS to look at the safety and effectiveness outcomes of AHSCT using either BEAM-ATG or Cyclophosphamide-ATG regimens. 41 of these patients were followed for greater than one year and included 34 with relapsing remitting MS and 7 with progressive forms of MS. The patients were followed for an average of close to 4 years with some followed for up to 9 years. At 5 years of follow-up 87% of participants had not experienced a relapse and 77% had not progressed based on EDSS scores, 68% had no disease activity (no relapses, no new MRI lesions and no disability progression). This study showed a significant difference in outcomes for patients who had gadolinium enhancing lesions prior to treatment compared to those who did not (79% of those with such lesions had no disease activity vs 46% in those who did not). No differences in efficacy were apparent between the two chemotherapy regimens, but the numbers in the Cyclophosphamide-ATG group were too small to make a reliable comparison. No transplant related deaths were recorded in this study and no patient required treatment in the intensive care unit. Almost all patients experienced acute toxicity during the hospitalisation period, with the expected side effects of hair loss, anaemia, low white blood cell counts and low platelet counts and half experienced infections. Later adverse events, following release from hospital, included reactivation of herpes zoster viral infections (8 patients) and thyroid disease (4 patients).
The three year interim data from the five year HALT-MS US trial was published in December 20149. 25 people with relapsing remitting MS were treated with a BEAM-ATG protocol. This study reported 78% of participants as ‘event-free’ after three years (defined as no relapse, no change in disability level and no new MRI lesions), and 58% at five years. At the three year time point, 91% of participants did not show any worsening of disability, whilst 86% of participants did not experience a clinical relapse. All participants experienced serious adverse events which required medical intervention in the first 100 days following transplant, most of which were related to blood and gastrointestinal systems. There was no transplant related mortality. Publication of the full results of this study is expected once five year follow-up has been completed for all patients.
The EBMTR has published a large case series of AHSCT provided for autoimmune disease between 1996 and 2007 including 345 people with MS1. A range of different chemotherapy regimens were used in this series, which also included people with relapsing remitting MS, secondary progressive MS and primary progressive MS. This data suggests that in approximately 55% of MS patients at three years of follow-up, inflammatory disease was halted with no evidence during the follow-up period of relapses, active lesions or disability progression. After 5 years, approximately 45% of people remained progression free (no lesions, relapses or disability progression)1. Overall 13 of 345 (3.7%) people with MS treated between 1996 and 2007 died of transplanted related causes1. In the acute 100 day post-transplant period, Transplant Related Mortality (TRM) was 2% for the entire series. However the 100 day TRM declined to 1.3% for people with MS treated between 2001 and 20077. TRM was closely related to the level of experience of the transplant centres1. The authors of the study were unable to draw any conclusions about the relative efficacy of the different chemotherapy regimens.
1. Farge D, Labopin M, Tyndall A, Fassas A, Mancardi GL, Van Laar J, Ouyang J, Kozak T, Moore J, Kötter I, Chesnel V, Marmont A, Gratwohl A, Saccardi R. Autologous hematopoietic stem cell transplantation for autoimmune diseases: an observational study on 12 years’ experience from the European Group for Blood and Marrow Transplantation Working Party on Autoimmune Diseases. Haematologica. 2010 Feb;95(2):284-92. doi: 10.3324/haematol.2009.013458. Epub 2009 Sep 22. [View abstract]
2. Snowden JA, Saccardi R, Allez M, Ardizzone S, Arnold R, Cervera R, Denton C,Hawkey C, Labopin M, Mancardi G, Martin R, Moore JJ, Passweg J, Peters C, Rabusin M, Rovira M, van Laar JM, Farge D; EBMT Autoimmune Disease Working Party (ADWP); Paediatric Diseases Working Party (PDWP). Haematopoietic SCT in severe autoimmune diseases: updated guidelines of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2012 Jun;47(6):770-90. doi:10.1038/bmt.2011.185. Epub 2011 Oct 17. [view abstract]
3. Pasquini MC, Voltarelli J, Atkins HL, Hamerschlak N, Zhong X, Ahn KW, Sullivan KM, Carrum G, Andrey J, Bredeson CN, Cairo M, Gale RP, Hahn T, Storek J, Horowitz MM, McSweeney PA, Griffith LM, Muraro PA, Pavletic SZ, Nash RA. Transplantation for autoimmune diseases in north and South America: a report of the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2012 Oct;18(10):1471-8. doi: 10.1016/j.bbmt.2012.06.003. Epub 2012 Jun 13. [view abstract]
4. Australasian Bone Marrow Transplant Recipient Registry 2014, Australasian Bone Marrow Transplant Recipient Registry: Annual Data Summary 2013, ABMTRR, Darlinghurst, NSW, Australia [more information here]
5. Majhail NS, Rizzo JD, Lee SJ, Aljurf M, Atsuta Y, Bonfim C, Burns LJ, Chaudhri N, Davies S, Okamoto S, Seber A, Socie G, Szer J, Van Lint MT, Wingard JR, Tichelli A; Center for International Blood and Marrow Transplant Research (CIBMTR); American Society for Blood and Marrow Transplantation (ASBMT); European Group for Blood and Marrow Transplantation (EBMT); Asia-Pacific Blood and Marrow Transplantation Group (APBMT); Bone Marrow Transplant Society of Australia and New Zealand (BMTSANZ); East Mediterranean Blood and Marrow Transplantation Group (EMBMT),; Sociedade Brasileira de Transplante de Medula Ossea (SBTMO. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012 Mar;18(3):348-71. doi: 10.1016/j.bbmt.2011.12.519. Epub 2011 Dec 13. [view abstract]
6. Mancardi GL, Sormani MP, Gualandi F, Saiz A, Carreras E, Merelli E, Donelli A,Lugaresi A, Di Bartolomeo P, Rottoli MR, Rambaldi A, Amato MP, Massacesi L, Di Gioia M, Vuolo L, Currò D, Roccatagliata L, Filippi M, Aguglia U, Iacopino P,Farge D, Saccardi R; ASTIMS Haemato-Neurological Collaborative Group, On behalf of the Autoimmune Disease Working Party (ADWP) of the European Group for Blood and Marrow Transplantation (EBMT); ASTIMS Haemato-Neurological Collaborative Group On behalf of the Autoimmune Disease Working Party ADWP of the European Group for Blood and Marrow Transplantation EBMT. Autologous hematopoietic stem cell transplantation in multiple sclerosis: a phase II trial. Neurology. 2015 Mar 10;84(10):981-8. doi: 10.1212/WNL.0000000000001329. Epub 2015 Feb 11. [view abstract]
7. Mancardi G, Saccardi R. Autologous haematopoietic stem-cell transplantation in multiple sclerosis. Lancet Neurol. 2008 Jul;7(7):626-36. doi:10.1016/S1474-4422(08)70138-8. Review. [view abstract]
8. Burman J, Iacobaeus E, Svenningsson A, Lycke J, Gunnarsson M, Nilsson P, Vrethem M, Fredrikson S, Martin C, Sandstedt A, Uggla B, Lenhoff S, Johansson JE, Isaksson C, Hägglund H, Carlson K, Fagius J. Autologous haematopoietic stem cell transplantation for aggressive multiple sclerosis: the Swedish experience. J Neurol Neurosurg Psychiatry. 2014 Oct;85(10):1116-21. doi: 10.1136/jnnp-2013-307207. Epub 2014 Feb 19. [view abstract]
9. Nash RA, Hutton GJ, Racke MK, Popat U, Devine SM, Griffith LM, Muraro PA, Openshaw H, Sayre PH, Stüve O, Arnold DL, Spychala ME, McConville KC, Harris KM, Phippard D, Georges GE, Wundes A, Kraft GH, Bowen JD. High-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for relapsing-remitting multiple sclerosis (HALT-MS): a 3-year interim report. JAMA Neurol. 2015 Feb;72(2):159-69. doi: 10.1001/jamaneurol.2014.3780. [view abstract]
10. Burt RK, Balabanov R, Han X, Sharrack B, Morgan A, Quigley K, Yaung K, Helenowski IB, Jovanovic B, Spahovic D, Arnautovic I, Lee DC, Benefield BC, Futterer S, Oliveira MC, Burman J. Association of nonmyeloablative hematopoietic stem cell transplantation with neurological disability in patients with relapsing-remitting multiple sclerosis.JAMA. 2015 Jan 20;313(3):275-84. doi: 10.1001/jama.2014.17986. [view abstract]
11. Shevchenko JL, Kuznetsov AN, Ionova TI, Melnichenko VY, Fedorenko DA, Kurbatova KA, Gorodokin GI, Novik AA. Long-term outcomes of autologous hematopoietic stem cell transplantation with reduced-intensity conditioning in multiple sclerosis: physician’s and patient’s perspectives. Ann Hematol. 2015 Jul;94(7):1149-57. doi: 10.1007/s00277-015-2337-8. Epub 2015 Feb 25. [view abstract]
12. Chen JT, Collins DL, Atkins HL, Freedman MS, Galal A, Arnold DL; Canadian MS BMT Study Group. Brain atrophy after immunoablation and stem cell transplantation in multiple sclerosis. Neurology. 2006 Jun 27;66(12):1935-7. [view abstract]
13. Roccatagliata L, Rocca M, Valsasina P, Bonzano L, Sormani M, Saccardi R, Mancardi G, Filippi M; Italian GITMO-NEURO Intergroup on Autologous Stem CellTransplantation. The long-term effect of AHSCT on MRI measures of MS evolution: a five-year follow-up study. Mult Scler. 2007 Sep;13(8):1068-70. Epub 2007 Apr 27. [view abstract]
14. Muraro PA, Robins H, Malhotra S, Howell M, Phippard D, Desmarais C, de Paula Alves Sousa A, Griffith LM, Lim N, Nash RA, Turka LA. T cell repertoire following autologous stem cell transplantation for multiple sclerosis. J Clin Invest. 2014 Mar;124(3):1168-72. doi: 10.1172/JCI71691. Epub 2014 Feb 17. [view abstract]
15. Loh Y, Oyama Y, Statkute L, Quigley K, Yaung K, Gonda E, Barr W, Jovanovic B,Craig R, Stefoski D, Cohen B, Burt RK. Development of a secondary autoimmune disorder after hematopoietic stem cell transplantation for autoimmune diseases:role of conditioning regimen used. Blood. 2007 Mar 15;109(6):2643-548. Epub 2006 [view abstract]
16. Darlington PJ, Touil T, Doucet JS, Gaucher D, Zeidan J, Gauchat D, Corsini R, Kim HJ, Duddy M, Jalili F, Arbour N, Kebir H, Chen J, Arnold DL, Bowman M, Antel J, Prat A, Freedman MS, Atkins H, Sekaly R, Cheynier R, Bar-Or A; Canadian MS/BMT Study Group. Diminished Th17 (not Th1) responses underlie multiple sclerosis disease abrogation after hematopoietic stem cell transplantation. Ann Neurol. 2013 Mar;73(3):341-54. doi: 10.1002/ana.23784. Epub 2013 Mar 5. [view abstract]
17. Sun W, Popat U, Hutton G, Zang YC, Krance R, Carrum G, Land GA, Heslop H, Brenner M, Zhang JZ. Characteristics of T-cell receptor repertoire and myelin-reactive T cells reconstituted from autologous haematopoietic stem-cell grafts in multiple sclerosis. Brain 2004 May;127(Pt 5):996-1008. Epub 2004 Feb 25. [view abstract]
18. Muraro PA, Douek DC, Packer A, Chung K, Guenaga FJ, Cassiani-Ingoni R, Campbell C, Memon S, Nagle JW, Hakim FT, Gress RE, McFarland HF, Burt RK, Martin R. Thymic output generates a new and diverse TCR repertoire after autologous stem cell transplantation in multiple sclerosis patients. J Exp Med. 2005 Mar 7;201(5):805-16. Epub 2005 Feb 28. [view abstract]
19. Rebeiro P, Moore J. The role of autologous haemopoietic stem cell transplantation in the treatment of autoimmune disorders. Intern Med J. 2015 Nov 2. doi: 10.1111/imj.12944. Epub ahead of print Review. [view abstract]
20 Correale J, Fiol M, Gilmore W. The risk of relapses in multiple sclerosis during systemic infections. Neurology. 2006 Aug 22;67(4):652-9. Epub 2006 Jul 26. PubMed PMID: 16870812. [view abstract]
21. Giacomo Boffa, MD; Luca Massacesi, MD; Matilde Inglese, MD, PhD; Alice Mariottini, MD; Marco Capobianco, MD; Moiola Lucia, MD; Maria Pia Amato, MD; Salvatore Cottone, MD; Francesca Gualandi, MD; Marco De Gobbi, MD; Raffaella Greco, MD; Rosanna Scimè, MD; Jessica Frau, MD; Giovanni Bosco Zimatore, MD; Antonio Bertolotto, MD; Giancarlo Comi, MD; Antonio Uccelli, MD; Alessio Signori, PhD; Emanuele Angelucci, MD; Chiara Innocenti, MD; Fabio Ciceri, MD; Anna Maria Repice, MD; Maria Pia Sormani, PhD; Riccardo Saccardi, MD; Gianluigi Mancardi, MD on behalf of the Italian BMT-MS study group. Long-Term Clinical Outcomes of Hematopoietic Stem Cell Transplantation in Multiple Sclerosis. Neurology, (2021) DOI: 10.1212/WNL.0000000000011461.
22. Das J, Snowden J, Burman J, et al. Autologous haematopoietic stem cell transplantation as a first-line disease-modifying therapy in patients with ‘aggressive’ multiple sclerosis. Multiple Sclerosis Journal. (2021). doi:10.1177/1352458520985238.
23. Mariottini A, Bulgarini G, Forci B, Innocenti C, Mealli F, Mattei A, Ceccarelli C, Repice AM, Barilaro A, Mechi C, Saccardi R, Massacesi L. Autologous haematopoietic stem cell transplantation versus low-dose immunosuppression in secondary-progressive multiple sclerosis. Eur J Neurol. (2022) Jun;29(6):1708-1718. doi: 10.1111/ene.15280.
24. Burt RK, Han X, Quigley K, Helenowski IB, Balabanov R. Real-world application of autologous hematopoietic stem cell transplantation in 507 patients with multiple sclerosis. J Neurol. (2022) May;269(5):2513-2526. doi: 10.1007/s00415-021-10820-2.
25. Jespersen F, Petersen SL, Andersen P, Sellebjerg F, Magyari M, Sørensen PS, Blinkenberg M. Autologous hematopoietic stem cell transplantation of patients with aggressive relapsing-remitting multiple sclerosis: Danish nation-wide experience. Mult Scler Relat Disord. (2023) Jun 13;76:104829. doi: 10.1016/j.msard.2023.104829.
26. Kalincik T, Sharmin S, Roos I, Freedman MS, Atkins H, Burman J, Massey J, Sutton I, Withers B, Macdonell R, Grigg A, Torkildsen Ø, Bo L, Lehmann AK, Havrdova EK, Krasulova E, Trnený M, Kozak T, van der Walt A, Butzkueven H, McCombe P, Skibina O, Lechner-Scott J, Willekens B, Cartechini E, Ozakbas S, Alroughani R, Kuhle J, Patti F, Duquette P, Lugaresi A, Khoury SJ, Slee M, Turkoglu R, Hodgkinson S, John N, Maimone D, Sa MJ, van Pesch V, Gerlach O, Laureys G, Van Hijfte L, Karabudak R, Spitaleri D, Csepany T, Gouider R, Castillo-Triviño T, Taylor B, Sharrack B, Snowden JA; MSBase Study Group Collaborators; MSBase Study Group Authors; Mrabet S, Garber J, Sanchez-Menoyo JL, Aguera-Morales E, Blanco Y, Al-Asmi A, Weinstock-Guttman B, Fragoso Y, de Gans K, Kermode A; MSBase Study Group. Comparative Effectiveness of Autologous Hematopoietic Stem Cell Transplant vs Fingolimod, Natalizumab, and Ocrelizumab in Highly Active Relapsing-Remitting Multiple Sclerosis. JAMA Neurol. 2023 Jul 1;80(7):702-713. doi: 10.1001/jamaneurol.2023.1184. [view abstract]
The National Health and Medical Research Council Resource for physicians Stem Cell Treatments – a Quick Guide for Medical Practitioners (2013).
The National Health and Medical Research Council Resource for patients considering stem cell treatment Stem Cell Treatments – Frequently Asked Questions (2013). These NHMRC resources cover all types of stem cell therapies and are not specific to AHSCT or MS. However, the considerations and suggested questions for patients to ask of treating centres are relevant for people who may be considering travelling overseas for AHSCT for MS.
The Stem Cells in MS Booklet produced through a collaboration of international MS societies including MS Research Australia and the MS International Federation (reprinted 2010).
The Australian Stem Cell Handbook – from Stem Cells Australia (last updated April 2015)